Routine Quality Assurance Protocol
A basic diagnostic imaging quality assurance program is a regulatory requirement in many provinces Footnote 1 Footnote 2 Footnote 3 Footnote 4 Footnote 5 and in federal institutions. 6-12 However there is currently no standardized QA test for CT AEC systems.
6-12 In addition standard AEC test objects generally consisting of acrylic blocks copper sheets or water phantoms are also available for such X-ray systems.
Routine quality assurance protocol. Quality Assurance in Software Testing. There are several reasons commonly given by those ho oppose initiating quality assurance testing forw ultrasound imaging equipment including the following. Testing has traditionally been the responsibility of Medical Physics Departments but the important role of sonographers has been recognised and recent publications have included tests to be performed by.
Adequate financial and logistical resources to. Quality Assurance QA plays an essential part in any analytical project. Accepted 11 February 2016.
Moore and Elizabeth J. This test methodology allows for continuing performance assessment of CT AEC systems and we recommend that this test should become part of routine CT quality assurance programs. The accuracy and integrity of these studies are of paramount importance to protecting human participants.
Which ISO 9000 defines as part of quality management focused on providing confidence that quality requirements will be fulfilled. Quality assurance focuses on improving the software development process and making it efficient and effective as per the quality standards defined for software products. Footnote 10 An ineffective quality assurance program can lead to poor quality radiograms that can impair diagnosis increase operating costs and contribute to unnecessary radiation exposure to both patients and.
Quality assurance programs in ultrasound has not yet been realized. Meaningful analysis of health facility data requires insights into the quality of the data. Ultrasound is considered to be an established and safe modality.
This report describes the QA test protocol performed at two institutions. Quality control is focused on fulfilling quality requirements and as related to clinical trials it encompasses the operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of the trial-related activities have been fulfilled. Procedures consistency in test results can be assured and trends monitored.
Quality Assurance James A. Routine cross checking-mechanisms D. The goal of routine retesting is to systematically assess intra-plate within the same plate and inter-plate on different plates variability.
Recipe for successful data quality assurance A. Effective QA ensures that decisions are made with an appropriate understanding of evidence and risks and helps analysts ensure the integrity of the analytical output. Numerous CT performance-evaluation procedures have been described in the literature but they are generally unsuitable for routine quality-assurance QA testing.
This Cookbook is a companion guide to the AIUM Routine Quality Assurance QA for Diagnostic Ultrasound Equipment document which outlines the basic QA requirements for AIUM- accredited practices. Quality ControlQuality Assurance QCQA can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements will be fulfilled. Introduction Purpose and Background.
The protocol is comprehensive and practical to. Therefore no quality assurance is necessary. This defect prevention in quality assurance differs subtly from defect detection and rejection in.
This provides a sense of how accurate and reliable the assay results are. PDF On Feb 1 1985 T D Cradduck and others published Use of NEMA protocols for routine quality assurance Find read and cite all the research you need on ResearchGate. Quality Assurance of ultrasound systems is necessary to ensure the reliability of results and to check for deterioration in performance.
Quality ControlQuality Assurance. It covers a wide range of matters that influence the quality of a product or service. Complete documentation of processes protocols readily available to collectors and processors C.
Quality assurance QA is a way of preventing mistakes and defects in manufactured products and avoiding problems when delivering products or services to customers. Clear strategy to respond to problems E. Routine quality control recommendations for nuclear medicine instrumentation.
The DQA toolkit includes an application for use in the DHIS2 for. Eur J Nucl Med Mol Imaging 2010 37662671 663. Routine QA ACR General US Program.
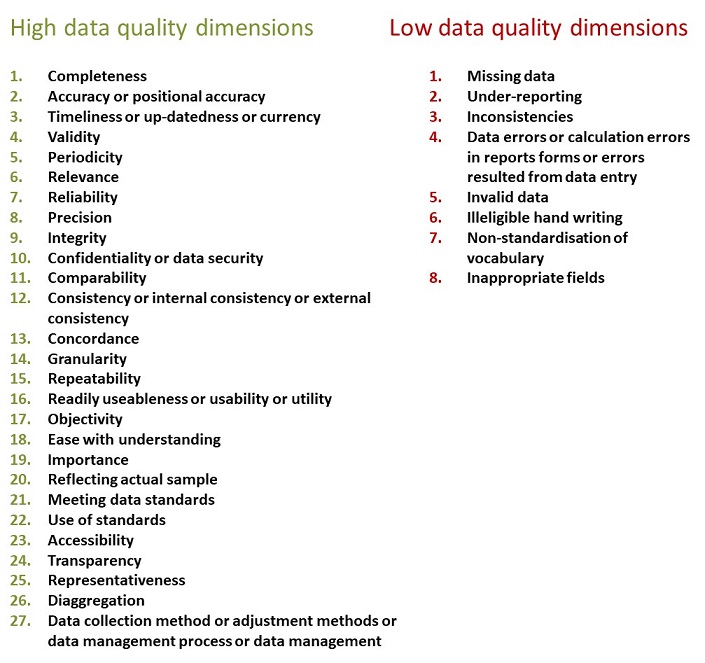
Tolerances of. Quality Assurance extends to all aspects of data collection from sanitary surveys to laboratory procedures. WHO has produced the Data Quality Assurance DQA toolkit to support countries in assessing and improving the quality of RHIS data.
Routine quality assurance QA tests for radiographic mammographic and fluoroscopic AEC systems are well established and documented in the literature. Quality Assurance and Quality Control Chapter 8 84 IPCC Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories 8 QUALITY ASSURANCE AND QUALITY CONTROL 81 INTRODUCTION An important goal of IPCC good practice guidance is to support the development of national greenhouse gas inventories that can be readily assessed in terms of quality and completeness. The Guide may be used as a brief step-by-step manual indicating when.
Quality Assurance in Software Testing is defined as a procedure to ensure the quality of software products or services provided to the customers by an organization. Quality Assurance for a recreational water monitoring programme will apart from helping to ensure that the results obtained are corr ect increase the confidence of funding bodies and the public. Yet the quality of Routine Health Information Systems RHIS data is an ongoing challenge in many contexts.
A number of bodies have produced guidelines. Can choose to do as many retests as desired but may want to keep. It is much more than getting the numbers right.
Includedi Includedi n some protocols but n some protocols but only briefly. A routine quality assurance test for CT automatic exposure control systems. The National Institute of Neurological Disorders and Stroke NINDS National Institutes of Health NIH currently supports over 1000 clinical research projects.
Received 27 October 2015. Adequate staff capacity supervision and accountability B. Routine Retesting for Quality Assurance.
But they do not specify the protocols to be followed which.
Clinical Trials And Regulatory Affairs Clinical Trials Journals Sciaeon

Points To Consider In Quality Control Method Validation And Transfer Bioprocess Internationalbioprocess International

Quality Assurance Quality Control Documentation Series Title
Quality Assurance For Small Scale Rural Food Industries Chapter 2 Quality Assurance Of Selected Commodities

Quality Assurance Checklist And Site Specific Information Form
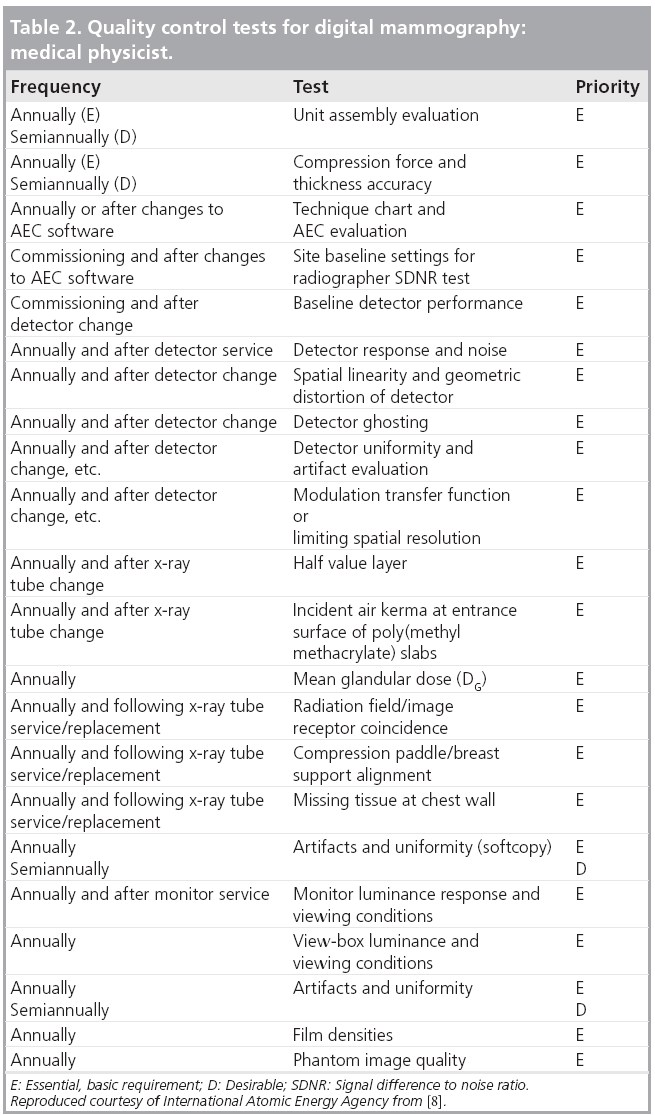
Developing A Quality Control Program For Digital Mammography Achievements So Far And Challenges To Come
Qa Program Questionnaire This Checklist Is Designed To Guide The Download Scientific Diagram
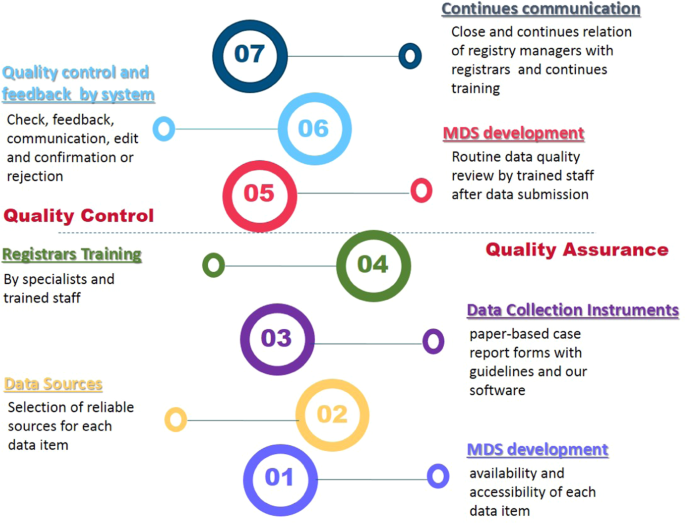
The Impact Of Data Quality Assurance And Control Solutions On The Completeness Accuracy And Consistency Of Data In A National Spinal Cord Injury Registry Of Iran Nscir Ir Spinal Cord Series And

Quality Assurance Qa Quality Control Qc Procedures Following The Download Scientific Diagram

Pdf Quality Assurance Of Computed And Digital Radiography Systems

Pdf Mri Quality Control Tools For Procedures And Analyses

Pdf Quality Assurance In Ct With The Belgian Protocol And The New European Acceptability Criteria

A Practical Implementation Of Physics Quality Assurance For Photon Adaptive Radiotherapy Sciencedirect

Pdf Application Of Qc Dr Software For Acceptance Testing And Routine Quality Control Of Direct Digital Radiography Systems Initial Experiences Using The Italian Association Of Physicist In Medicine Quality Control Protocol

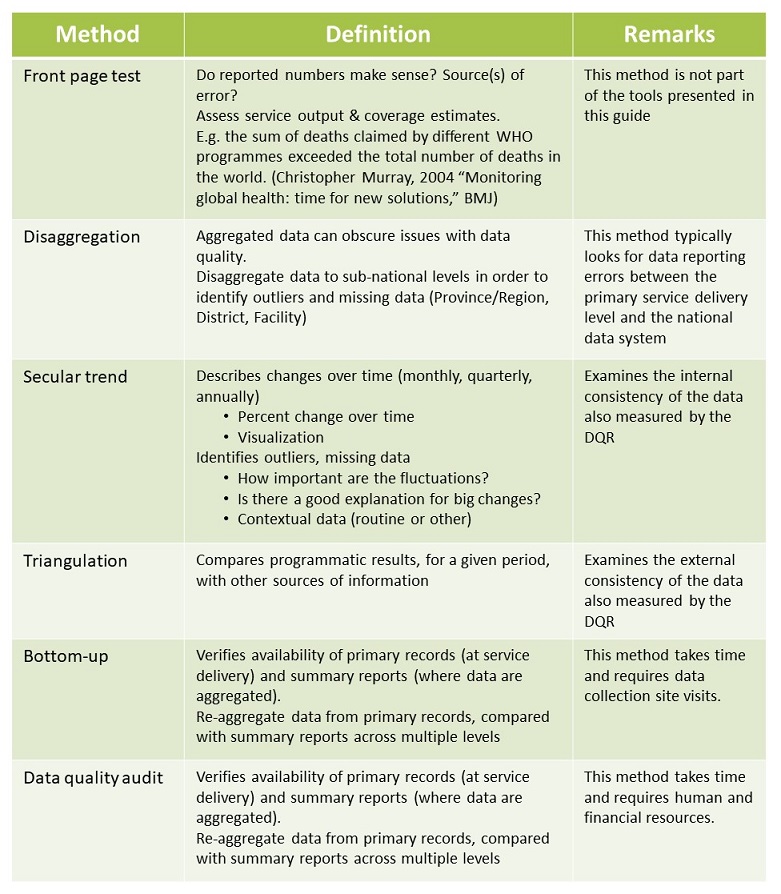
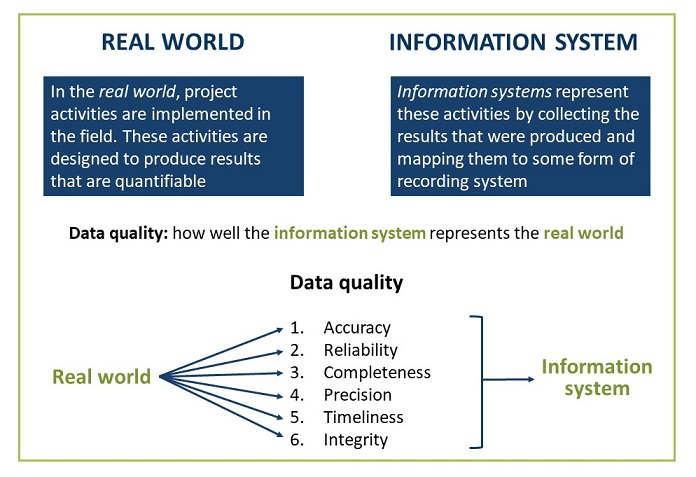
Posting Komentar untuk "Routine Quality Assurance Protocol"