Definition Of Quality Assurance In Pharmaceutical Industry
Quality Assurance Department Functions in Pharmaceuticals. She has gained experience in Quality Assurance Quality Systems QA QS by completing work in both pharmacy and the food industry.
Definitions Principles Of Good Manufacturing Practice Pharmaceutical Microbiology
QC department has a set of specified tests to ensure drug quality and purity.

Definition of quality assurance in pharmaceutical industry. Current Good Manufacturing Practice cGMP Good Laboratory Practice GLP GCP etc and local. Every person working in pharmaceuticals should always care about the product quality. Q10 Pharmaceutical Quality System.
Quality Assurance What is Role of Quality Assurance department in Pharmaceutical Industry. Qc is the part of GMP concerned with sampling specifications and testing and with the organization documentation and release procedures which ensure that the necessary and relevant tests are actually carried out and the materials are neither released for use nor products are used for sale supply until their quality has been satisfactory. Anastasia has worked in Greece in the food industry as a Quality Assurance technician and in the UK pharmaceutical industry at Norbrook Laboratories Ltd in Northern Ireland and gained experience in testing raw materials as a Quality Control Analyst.
Appropriate quality assurance is important in the pharmaceutical industry. Without it companies cannot guarantee that their products conform to the appropriate standards for quality and safety. Quality assurance for pharmaceuticals Summary 192 191Pharmaceutical quality 192 Pharmaceutical quality assurance framework Defining and assessing pharmaceutical quality Consequences of poor pharmaceutical quality Determinants of pharmaceutical quality Prevalence of poor-quality.
A compendium of guidelines and related materials. PHARMACEUTICAL QUALITY ASSURANCE Acknowledgment Material in session 5 is adapted from Management Sciences for Healths Managing Drug Supply chapter 18 Quality Assurance for Drug Procurement MSH 1997. Quality Control is a testing unit in the pharmaceutical.
Definition-ICH -ICH Good Clinical Practice Definition of Quality AssuranceThe planned and systematic actions that are established to ensure that the trial is. Quality Assurance Structure QAS plays an essential role in the profitability and market size of pharmaceutical companies. Once a drug product manufactured need to test and approve the drug as per predefined regulatory standards.
Quality assurance of pharmaceuticals. Quality Assurance is one of the department in the pharmaceutical manufacturing industry. There should not be any compromise with quality.
Quality Control in Pharmaceutical Industry 1. Performed and the data are generated documented and recorded in compliance with Good Clinical Practice and applicable regulatory. In the pharmaceutical industry quality assurance QA is essential for ensuring that pharmaceutical products are manufactured to a safe and consistent standard.
Performed and the data are generated documented and recorded in compliance with Good Clinical Practice and applicable regulatory requirements. QA is a very broad field that refers to any aspect that may affect a drugs quality during its research development manufacturing and sales phases. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research CDER.
Guidance for Industry. ICH Good Clinical Practice Definition of Quality Assurance. Purpose and Content The purpose of quality assurance QA in public pharmaceutical supply systems is to make.
The concept of quality assurance and quality control together develops towards assuring the quality. It enforces compliance with US FDA current Good Manufacturing Practice cGMP ISO 90012015 and other regulatory requirements and standards concerned. Accordingly this study aimed to evaluate the quality.
ICH Q10 - Pharmaceutical Quality System The pharmaceutical quality system assures that the desired product quality is routinely met suitable process performance is achieved the set of. Quality of the pharmaceutical must be the most important thing for pharmaceutical companies. The planned and systematic actions that are established to ensure that the trial is.
Pharmaceutical Quality Management System Pharma QMS A quality management system helps pharmaceutical organizations ensure the quality of products and process improvements. Quality Assurance Department shall function for assuring the quality of all the Products manufactured at every stage of manufacturing processing of Drug Products. 2 Good manufacturing practices and inspection.
It is an obligation that ensures manufacturers meet the needs of end-user needs in terms of safety quality efficacy strength reliability and durability. Quality assurance is a good practice in the manufacture of pharmaceutical products as it is the process of vouching for integrity of products to meet the standard for the proposed use. The quality assurance department must operate independently from the operational units and it must regularly perform quality review activities self-inspection auditsinternal audits to ensure compliance within operational units with Company quality standards good working practices GxPs.
Quality Assurance is assuring the quality of the drug product manufactured in the facility by implementing and following the compliance in the unit. What is Role of Quality Assurance department in Pharmaceutical Industry. Quality Control is a sum of all procedures undertaken to ensure the Quality Identity and Purity of the.
Although everyone in a company is ultimately responsible for quality executives and other members of top management have an important responsibility. 1Drug and narcotic control standards 2Drug industry standards 3Pharmaceutical preparations standards 4Biological products standards. It is a matter of feeling and the definition varies from person to person depending on the perspective in.
This shall be achieved by performing the functions of monitoring as per the laid systems for the following areas which. Quality assurance total quality control total quality management TQM pharmaceutical.

An Overview Of Quality Assurance In Pharmaceutical Industry Youtube

What Is Quality Assurance Definition And Examples
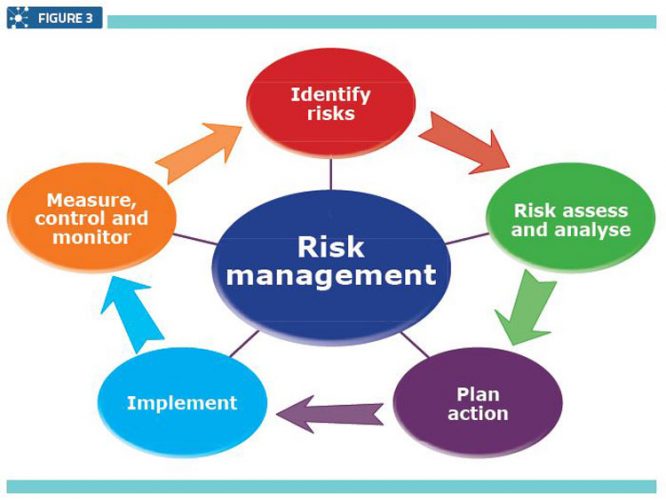
Quality Assurance Pharmaceutical Quality Systems In Making Medicines
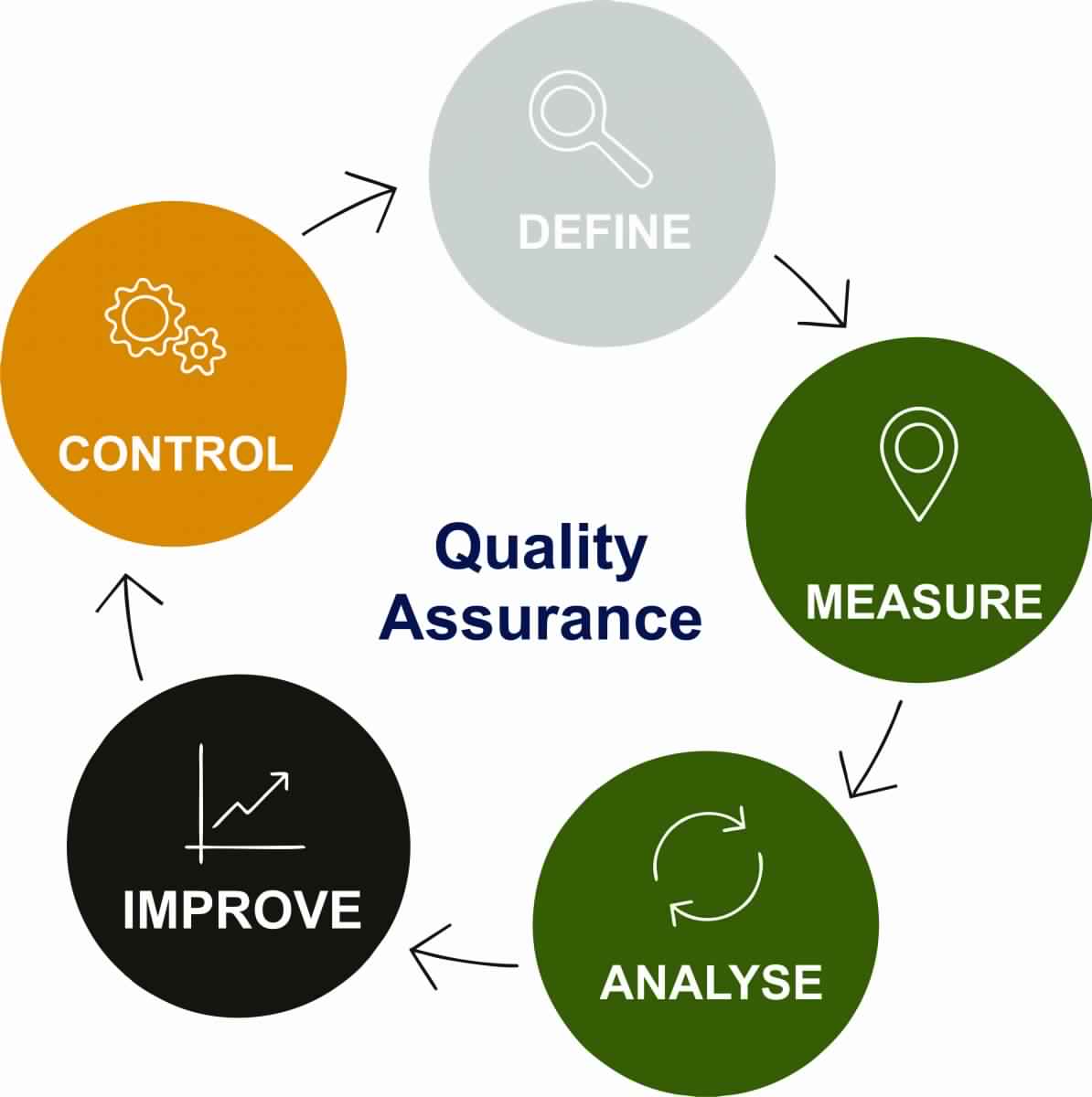
Quality Assurance Pharma Advisor
Pharmaceutical Quality Assurance Fda S Quality Unit Expectations
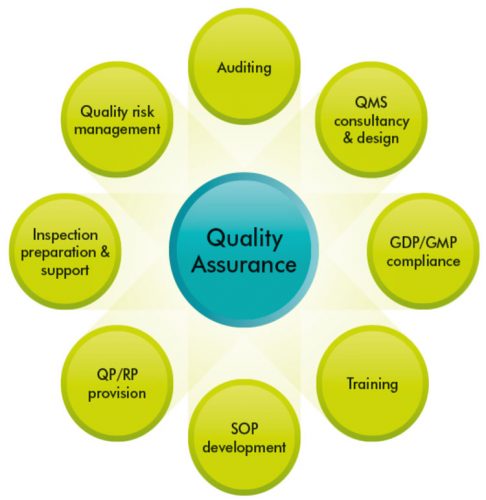
Quality Assurance Pharmaceutical Quality Systems In Making Medicines
Role Of Qa And Gmp In Pharma Industry Apex University

Quality Assurance Vs Quality Control Chapter 5 Online Presentation

100 Quality Assurance Interview Questions Pharma Pharmaguddu

Difference Between Quality Assurance And Quality Control Pharmaceutical Guidelines

Ppt Pharmaceutical Quality Assurance Powerpoint Presentation Free Download Id 3878460
Quality Assurance Qa Management Procedures Quality Assurance
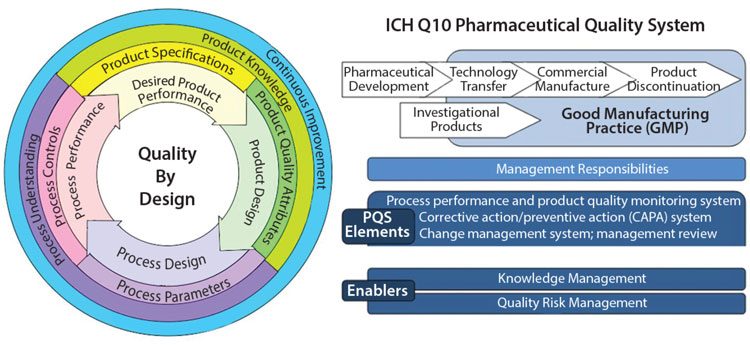
Quality Assurance Pharmaceutical Quality Systems In Making Medicines

Quality Metrics For Pharmaceutical Manufacturing Pharmaceutical Guidelines
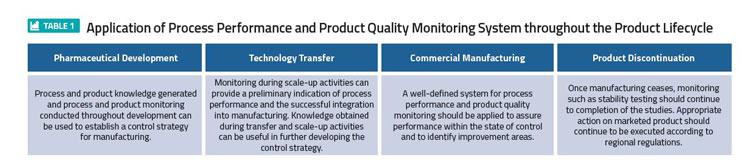
Quality Assurance Pharmaceutical Quality Systems In Making Medicines

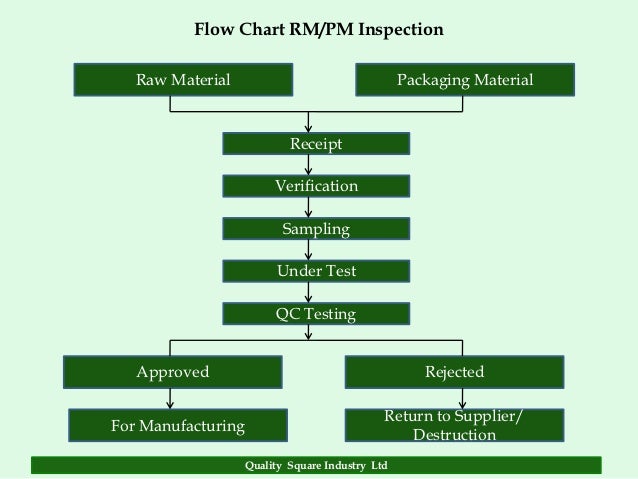


Posting Komentar untuk "Definition Of Quality Assurance In Pharmaceutical Industry"